Determine Which Reaction Has the Highest Activation Energy.
This cannot be determined from the given information. The rate constant for the reaction H 2 g I 2 g --- 2HIg is 54 x 10-4 M-1 s-1 at 326 o C.
We therefore start by calculating 1T and the natural logarithm of the rate constants.

. Catalysts increase the rates of reactions by providing a new mechanism that has a smaller activation energy as shown in the figure below. All reactions are activated processes. Each of the above steps offers resistance to.
Determine which reaction has the highest activation energy. 2077 E a 452 x 10 -5 molJ E a 459 x 10 4 Jmol. Activation energy is ___.
A larger proportion of the collisions that occur between reactants now have enough energy to overcome the activation energy for the reaction. The slowest step in the reaction mechanism has the highest activation energy. Determine which reaction has the highest activation energy.
An activation energy is a potential energy barrier that reactants have to get over in order to react. At 410 o C the rate constant was found to be 28 x 10-2 M-1 s-1. A b c d.
Reacting metal and acid. Which reaction has the higher activation energy. B Which of the reactions.
In lab you will record the reaction rate at four different temperatures to determine the activation energy of the rate-determining step for the reaction run last week. The energy needed to overcome the activation energy comes from the kinetic energy of random collisions. E a the activation energy of the reaction in Jmol R the ideal gas constant 83145 JKmol T 1 and T 2 absolute temperatures Kelvin k 1 and k 2 the reaction rate constants at T 1 and T 2.
Here is more about this topic in the following video. Formula to calculate activation energy. Kinetics of liquid-particle reactions in hydrometallurgy.
Determine which reaction has the highest activation energy. The first step is the slow step since it has the highest activation energy. If there is an early step with a low transition state the intermediate will accumulate the forward and reverse reaction of the first step achieve equilibrium and the overall reaction will depend on the later.
Which physical state of nitrogen has the highest entropy. We can determine the activation energy for a reaction from a plot of the natural log of the rate constants versus the reciprocal of the absolute temperature. Correct answer - Determine which reaction has the highest activation energy.
It is characterized by its high activation energy. To determine activation energy of a reaction Ea you need the reaction rate constants at different temperature. Answer 1 of 6.
The barrel reeks of an. In the following image which reverse reaction has the highest activation energy. Determine the activation energy of the reaction in kJmol where the rate of a certain reaction multiplies by 3609 when the temperature changes from 105C 37815K to 168C 44115K.
A chemist is summoned to an abandoned waste-disposal site to determine the contents of a leaking corroded barrel. In reverse reaction reaction starts from G so it can be seen clearly that most of activation energy is needed from product E to D. Reaction B has the higher activation energy.
The two factors determine whether a reaction is. Reacting metal and acid B. In endothermic reactions the final products have a higher energy than the reactants.
Reacting metal and acid B. Check all that apply. Calculate the a activation energy and b high temperature limiting rate constant for this reaction.
The energy that is available to do work in a reaction is called ____. How to determine order and activation energy. Reacting metal and acid C.
An energy diagram is shown below Figure 125 for the endothermic reaction. The rate determining step in a reaction mechanism is the slowest step. If a reaction has an equilibrium constant just greater than 1 what type of reaction is it.
A Which of the reactions in the figure above has the highest activation energy for the reverse reaction. The rate-determining step is the step which leads to the transition state with the highest overall energy. 28 March 2022 by Huwas.
It follows naturally that the step with the highest activation energy will be the slowest step. That is what makes it slow and why it is called the rate determining step. From the Arrhenius equation a plot of lnk vs.
O 2 g N 2 g 2 NO g Notice that the activation energy for the endothermic reaction is much greater than for the exothermic reaction. As a result the rate of reaction increases. Chemical Kinetics Reaction Mechanism.
All chemical reactions including exothermic reactions need activation energy to get started. Reaction rate constant is the connection between rate and concentration or in formula r kAB in case of a second order. Or in kJmol divide by 1000 E a 459 kJmol.
From the Wade textbook on kinetics and activation energy. What will be the ratio of the rate at 105C 37815 and the rate at 40C 31315K. Chemistry questions and answers.
The higher this potential the slower the reaction the exact relationship is shown by the Arrhenius equation. 1T will have a slope m equal to EaR. The activation energy for.
Determine which reaction has the highest activation energy. R in this case should match the units of activation energy R 8314 JK mol. Consider the energy diagram represented below of a two-step mechanism.
What Is The Activation Energy For A Reverse Reaction Quora
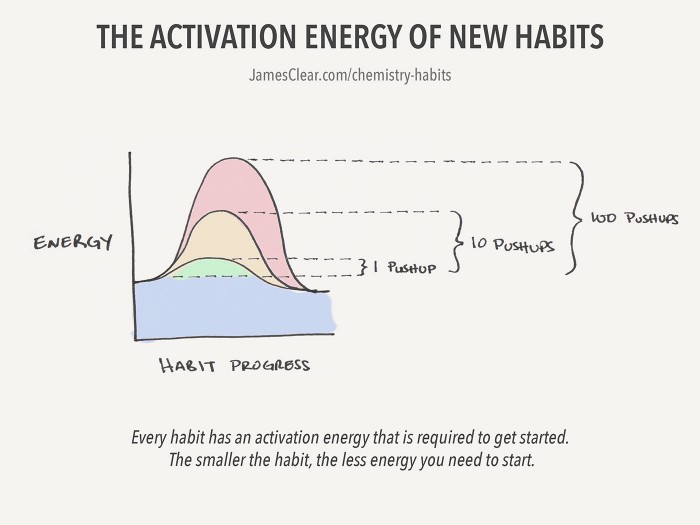
Activation Energy And The Chemistry Of Building Better Habits

Activation Energy Article Khan Academy
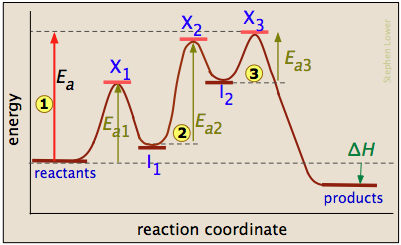
Reaction Mechanisms And Multistep Reactions Chemistry Libretexts

7 6 Energetics And Kinetics Chemistry Libretexts
Illustrated Glossary Of Organic Chemistry Rate Determing Step

12 5 Collision Theory Chemistry

18 4 Potential Energy Diagrams Chemistry Libretexts

Multi Step Reaction Ck 12 Foundation
Activation Energy And Temperature Dependence Boundless Chemistry

Activation Energy Article Khan Academy

16 1 Rate Determining Step Hl Youtube
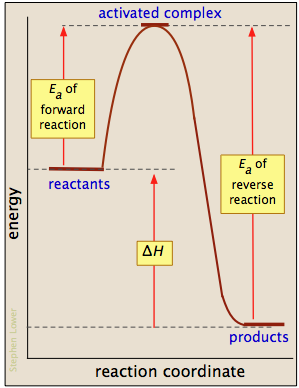
If Reaction A B Is Exothermic How Does The Activation Energy For The Forward Reaction Compare With The Activation Energy For The Reverse Reaction B A Socratic

Question Video Identifying Steps In A Reaction Profile Diagram For A Two Step Chemical Reaction Nagwa

Chemical Equilibrium And Kinetics For The Mcat Everything You Need To Know Shemmassian Academic Consulting
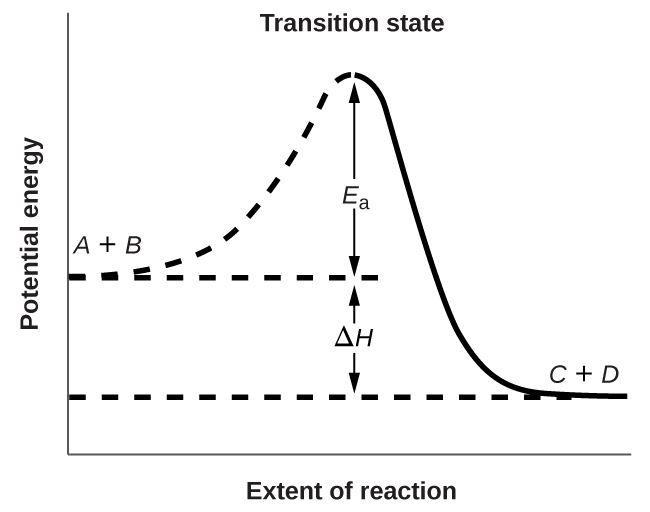
12 5 Collision Theory Chemistry
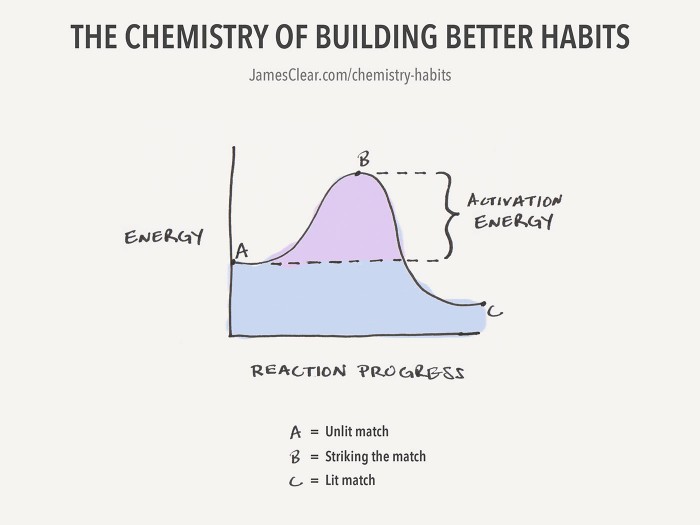
Activation Energy And The Chemistry Of Building Better Habits
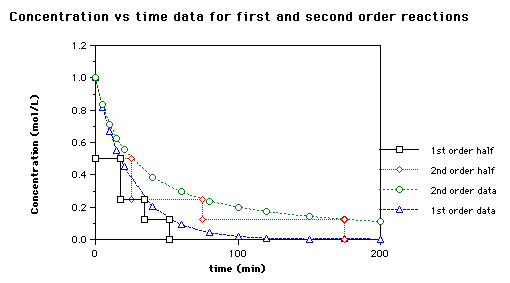

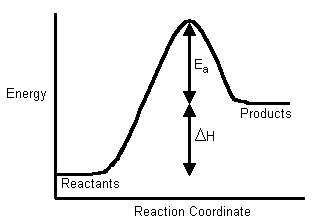
Comments
Post a Comment